Downsloping
Hello everyone!
A great example of downsloping is underway this morning across the northeast as northwesterly winds are in place behind a cold front that moved offshore last night. That means that today is a great day to talk about downsloping, also known as adiabatic warming. So what is downsloping, how does it work, and how does it affect your weather? Downsloping is the process that occurs when a stream of air is forced to descend a mountain. As the air descends, it undergoes a series of changes that result in a warming/drying effect. This series of changes is known as adiabatic warming. So what does that mean? Adiabatic means the process involves no outside transfer of heat from the surrounding environment to one of the air “parcels” in that descending stream. An air parcel is just some chunk of air in that stream that we’re going to pick out and track as it undergoes the process we’re looking at. With that in mind, let’s take a visual look at the downsloping process
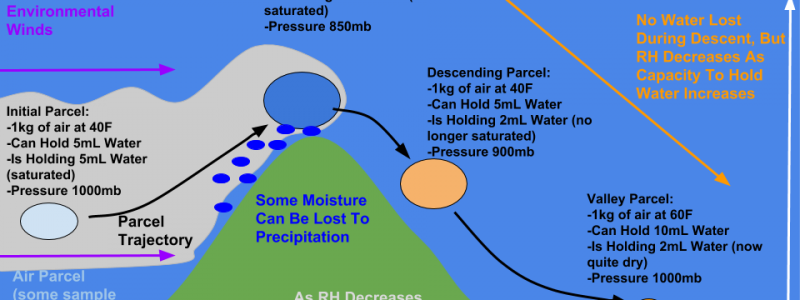
First a quick note that these values are approximate and just for illustrative purposes. They could be calculated exactly using a series of relationships and equations, but that’s beside the point. I tried to make them roughly accurate, but anyone who wants to do out the calculations can feel free to correct any value that is a bit off. To illustrate the process, we’re going to follow a 1kg parcel of air upwind of a mountain. It has the capacity to hold 5mL of water, and in fact is filled to the brim. We call this saturation, and a parcel at saturation will produce a cloud. As this parcel is forced to rise up the mountain, it cools. As it cools, its capacity to hold water decreases (cold air can’t hold as much moisture as warm air). Some water usually falls out of the cloud as a result, in the form of rain, snow, or drizzle. Other times, this process (known as upsloping) will simply bring the air to saturation, creating a cloud but no precipitation. At the top of the mountain, the parcel has a lower pressure, and thus a higher volume. It also has a lower temperature, and can only hold 2mL of water. As it crests the ridge, the downsloping process begins.
As the parcel descends, its pressure increases. That makes sense because as you move lower down in the atmosphere, there’s more air above you to press down on you. As the pressure increases, the parcel shrinks in volume (look to the ideal gas law PV=nRT for a mathematical proof of this). The physical act of the parcel shrinking involves the outside environment “doing work” (in the physics sense of the phrase) on the parcel to fit it into a smaller space (think of crumpling up a piece of paper, or packing together a snowball). This work ends up raising the temperature of the parcel, not due to an addition of heat energy, but instead as a result of an application of mechanical energy. So how does this physics and chemistry result in sensible weather changes?
As the parcel warms, its capacity to hold water vapor increases. Instead of being able to hold only 2mL when it was at 20F at the summit, it can now hold 10mL when it’s 60F in the valley below. It hasn’t lost any of its actual water vapor from the summit (it still has 2mL), but its water content relative to its potential water content is now very low (it’s only 20% “full” instead of 100%). This is what we talk about with Relative Humidity. The descending air has a very low RH, even though it hasn’t lost any of its actual water vapor. To have clouds, you need very high RH values around 100%. So as the RH drops downwind of the mountain, the clouds dissipate!
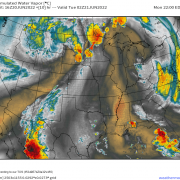
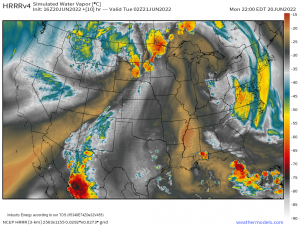
Here’s an example of this process in action across the Northeast. Note the low cloud deck NW of the Appalachian mountains which all of the sudden evaporates as it descends towards the coastal plain. If you live in the I-95 corridor, downsloping is what gives you these beautiful sunny days while inland areas are stuck with overcast. Hooray for adiabatic warming!
Downsloping occurs anywhere you have mountains, and can actually have long-term climate effects.
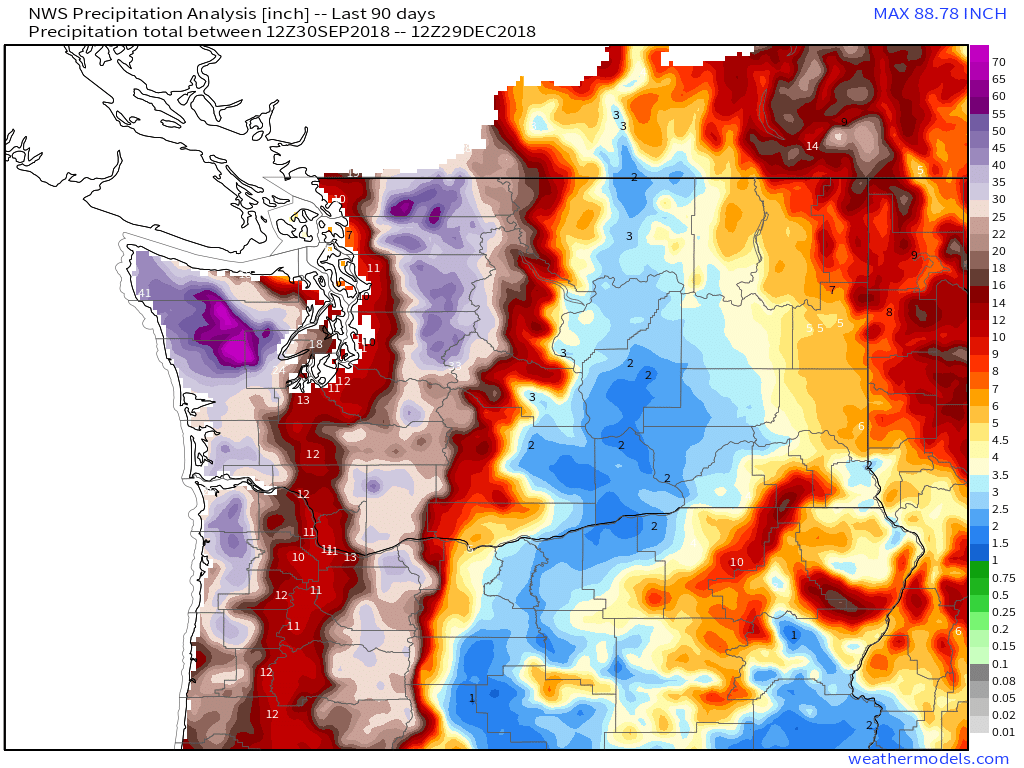
For example, downsloping off the Cascades produces dramatic reductions in rainfall as Pacific storms move ashore in Washington State. Over the past three months, the peaks have seen 50-70″ of precipitation as the mountains squeeze moisture out of the air via upsloping. As the air descends the eastern slopes of the mountains, adiabatic warming dries it out and very little if any precipitation falls. Thus parts of Eastern Washington have seen only 2-3″ of precipitation in the same three month timeframe. The result is lush forests east of the mountains, with a dry and arid climate to the west as the same process plays out year after year after year. The same dynamic also plays out in California with the Sierra Nevada where the ridgeline separates the climate of the fertile Central Valley from the deserts of Nevada.
-Jack






Happy New Years from Not-so-Frosty in Southern NH,
Thanks for explaining down-sloping in this post. Occasionally, I hear about a pressure build against the upslope sides of mountains, which should raise temperature (some, a tad, a bit?) relative to a blob of air not pushed against a mountain. What, if any, significant effect does this produce? And I suspect there’s some corresponding pressure decrease on the back side of the mountain, perhaps creating some kind of air eddy?
Happy New Years to you as well! Yes, upslope flow generates a little bubble of high pressure as the air is crammed against the mountain, but this effect is very small relative to the pressure change by going up or down in the atmosphere. The pressure might increase from 1010mb to 1016mb in a pretty strong case, but the parcels in that little high pressure bubble are then forced upward to (in the case of the White Mountains) about 800mb. The temperature drop due to the lifting (200mb change) overwhelms the temperature increase from the localized high pressure (16mb change). Hope that helps!